Gmos and genome edited organisms (Geos): regulatory and geopolitical challenges
Introduction
GMOs : discrepancies around the globe
Biotech crops are gaining ground
A great global divide
Developing countries lead in biotech crops
A race for innovation in a heterogeneous regulatory world
Genome editing organisms (GEOs): the race for innovation and patenting
European regulations steeped in the principle of precaution
High stakes: power and progress in the battle for social acceptability
Conclusion
Summary
Methods of genome modification are intimately linked to the history of mankind, from the early beginnings of agriculture in the Neolithic period to the development of gene therapies throughout the 20th century. Today, the techniques used are derived from biotechnologies (transgenesis, mutagenesis, and new genome editing techniques known as NBT, for new breeding techniques) and have sparked a societal debate riddled with mistrust or ideological rejection.
Are these fears founded?
At the very least, the scientific advances and opportunities offered by these biotechnologies warrant our consideration, especially in the health sector. Today, globalisation means that health, whether human, animal, plant or environmental, is interdependent and can be referred to collectively under the concept of One Health, One World.
In this study, we examine the current and future regulatory issues concerning genetically modified organisms (GMOs) and genome edited organisms (GEOs), as well as the geopolitical impact in certain countries resulting from the mistrust triggered by the emergence of these biotechnologies.
Catherine Regnault-Roger,
Emeritus University Professor at the University of Pau and Pays de l'Adour (Iprem –e2s UPPA), member of the French Academy of Agriculture, corresponding member of the National Academy of Pharmacy, member of the Scientific Committee of the High Council for Biotechnology.

Catherine Regnault-Roger,
Emeritus University Professor at the University of Pau and Pays de l'Adour (Iprem –e2s UPPA), member of the French Academy of Agriculture, corresponding member of the National Academy of Pharmacy, member of the Scientific Committee of the High Council for Biotechnology.
François Rabelais, Pantagruel, VIII, in Œuvres complètes, Gallimard, “Bibliothèque de la Pléiade”, 1994, p. 245.
“Le regard des Français sur l’Agriculture. Résultats“, Ipsos survey for Opinion Valley, October 2018, p. 9. Genetically modified organisms (GMOs) and genetically modified plants will also be referred to here as biotechnological plants or biotech plants.
See Catherine Regnault-Roger, Des outils de modification du génome au service de la santé humaine et animale and Des plantes biotech au service de la santé du végétal et de l’environnement, Fondation pour l’innovation politique, January 2020.
“Science without conscience is but the ruin of the soul,” Rabelais wrote as early as the 16th century1. This judgment by Gargantua for the education of his son Pantagruel is undoubtedly the best representation of the state of mind in the controversy around technological advances and the progress they generate for society, sparked in the 20th century and still raging more than ever today. This generalised mistrust affects fields as varied as physics, with nuclear power, chemistry, with synthetic organic pesticides or petroleum-derived plastics, but also biology, through genome modification techniques that took off during the 20th century under the name of “biotechnology”.
The use of these biotechnologies to improve plant health has been at the centre of controversy for some forty years, and is now crystallising the debate to such an extent that in 2018, 75% of our fellow citizens tried to ensure that their food did not contain any genetically modified organisms (GMOs)2, an attitude that, at best, reflects widespread suspicion. Undoubtedly, they must assume that GMOs are harmful to their health, though these widespread suspicions have no basis in scientific fact. On the contrary, GMOs and genome edited organisms (GEOs), resulting from New Breeding Techniques (NBT), are proving to be useful tools for human, animal and crop health as well as for the preservation of the environment and adaptation to climate change3.
This mistrust of GMOs has led the world to split into two camps: on one side, the countries that have adopted biotechnology; on the other, those that have rejected it, at least in the agricultural field. What does that map of the world look like and how will it evolve? This work will examine the role regulation has played in the current state of GMOs in the world and the mistrust they inspire. It is indeed crucial to identify the context in which GMO acceptance or rejection occurs and the demands of consumers. Since the introduction of the first regulatory measures in the European Union, scientific knowledge has made significant progress. Consequently, it is now relevant and legitimate to ask whether European regulation is still adequate considering the risks involved, and what the geopolitical stakes of future regulation of GEOs are. These are all questions which we will try to provide the answers to based on scientific realities.
GMOs : discrepancies around the globe
Biotech crops are gaining ground
See Catherine Regnault-Roger, Des plantes biotech…, op. cit.
See “Global Status of Commercialized Biotech/GM Crops in 2017 : Biotech Crop Adoption Surges as Economic Benefits Accumulate in 22 Years“, ISAA Briefs, n°53, 2017.
See “Global Status of Commercialized Biotech/GM Crops in 2017: Biotech Crops Continue to Help Meet the Challenges of Increased Population and Climate Change”, ISAAA Briefs, n°54, 2018.
Between 1984 and 1995, many field experiments (25,000) preceded the cultivation and commercialisation of genetically modified plants (GMPs) derived from transgenesis4. Forty-five countries hosted these field trials, but nearly three-quarters of them were conducted in the United States and Canada, while the remaining 7,000 trials were conducted in Europe, South America, Asia and South Africa5. Following the approval of these trials, in 1996, five countries embarked on an adventure to grow GMPs: the United States, Canada, Argentina, Australia and Mexico, thus joining a sixth country, China, a pioneer that had already marketed transgenic tobacco and tomatoes as early as the beginning of the decade. In 1996, transgenic plants covered 1.7 million hectares, 51% of which was in the United States and 39% in China. Canada and Argentina each grew 4%, and Australia and Mexico less than 1%. In 1997, the area covered with transgenic plants had increased to 11 million hectares, a six-fold increase from the previous year. Ten years later, in 2007, the area covered by biotech crops stood at 114.3 million hectares, an increase of 105.1 million hectares. This growth trend was sustained and, in 2018, the latest available statistics revealed that there are 191.7 million hectares6 of transgenic crops, an increase of more than 77 million hectares over the last decade.
Yet not all countries grow biotech plants. In fact, the world is divided into two subsets: the regions that cultivate them and the others that do not. On the one hand, there is the American continent and the Asian continent as well as Oceania; on the other, the European continent (apart from a few countries, such as Spain and Portugal), including the Russian Federation, the Middle East and many African countries.
A great global divide
The American continent at the forefront of progress
The American continent as a whole, North and South, accounts for 87% of the land cultivated with GM crops. The United States is the undisputed leader with the largest surface area since 1996: 75 million hectares have been exploited. It is also the country with the greatest diversity of transgenic crops on the market and the largest number of trials. Corn (45% of the 2017 acreage is biotech), soybeans (45%), cotton (6%), alfalfa (2%) and canola (1%) are most prevalent, while more marginal crops include: sugar beet, papaya, squash, Arctic® apples and Innate® potatoes. Since 1996, over a period of 20 years, the United States approved 197 transformation events in 19 crop species including 43 for maize, 43 for potatoes and 25 for soybeans. These crops were produced by 420,000 farmers.
Major biotech plant producing countries (2007-2017)
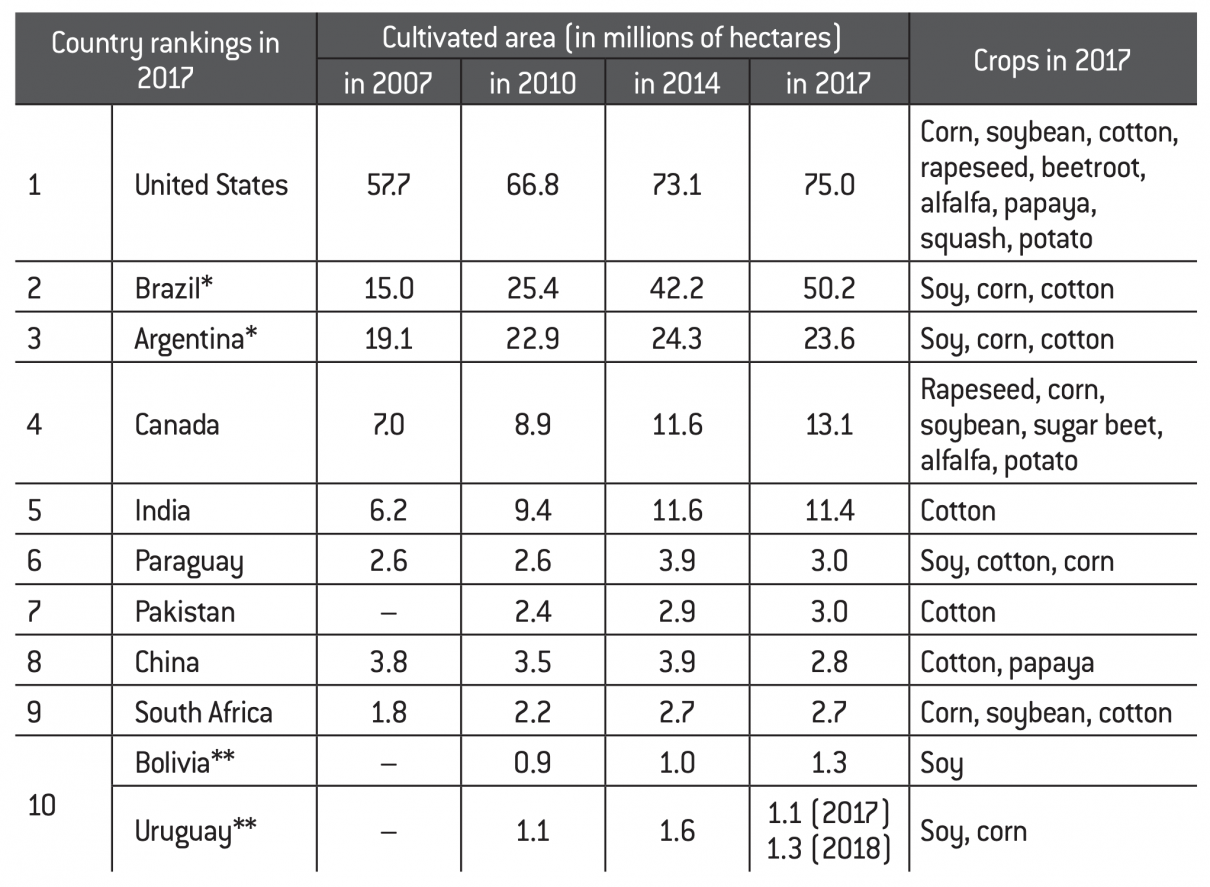
Source :
Fondation pour l’innovation politique; data from various ISAAA annual reports.
Brazil and Argentina swapped places in the rankings in 2010.
Bolivia and Uruguay are tied in 2018 with 1.3 million hectares (due to a temporary decrease in cultivation of 200,000 hectares in Uruguay in 2017 alone).
Canola is genetically modified rapeseed produced in See Canola Council of Canada, “Canola : Myths debunked”, canolacouncil.org, n.d.
Stacking means that there are several transformation events (in this case, three) on the same plant. See Catherine Regnault-Roger, Des plantes biotech…, op. cit.
Herbicide Tolerant: HT; Insect Resistant: IR.
See Catherine Regnault-Roger, Des plantes biotech…, op. cit.
See “Global Status of Commercialized Biotech/GM Crops in 2017…”, op. cit.
Canada was the first country to allow the commercialisation of canola (GM7 rapeseed) in 1996. The country currently ranks fourth in terms of area under GMP cultivation (12.75 million hectares in 2018) and shows great dynamism. Its main crops are canola, grown on 67% of the GMP area, followed by soybean (19%) and corn (13.5%). The cultivation of GM sugar beet (15,000 hectares), alfalfa (4,000 hectares) and potatoes (65 hectares) is more marginal. Since 1996, 177 processing events have been approved by Canadian authorities, recently even threefold8 for corn and potatoes. Foods from GM crops, such as Artic® apples, sugar cane and golden rice, have been approved for consumption.
Latin American countries are not to be underestimated in this field, as some, such as Mexico, Argentina and Uruguay were among the pioneer countries hosting early trials between 1984 and 1995, and had their first crops in the field in 1996.
In Argentina, where there are 18 million hectares of GM crops, biotech soybeans account for 75% of the 23.9 million GM hectares exploited, followed by biotech maize (5.5 million hectares) and, to a lesser extent, GM cotton (370,000 hectares, up 60% between 2017 and 2018). These crops cover more than half (60%) of the country’s arable land. All soybeans grown are GM and the adoption rate of biotech corn is 97%. Most varieties grown are Herbicide Tolerant9 (83% of soybeans are HT) or polyvalent IR-HT (83% of corn). There has been a strong increase in HT-IR Intacta™ soybeans which occupied 70,000 hectares in 2015 and expanded to 3.08 million hectares in 2017. New transformation events have been developed by the Agricultural Biotechnology Institute of Rosario to adapt crops to drought and salinity, approved in 2015, and for a low lignin GM alfalfa that increases feed digestibility, approved in 2018 (accounting for only the third worldwide approval granted, after those in the United States and Canada).
Brazil started growing GM crops in 2003, later than Argentina, but has since supplanted it devoting 51.3 million hectares to biotech crops (more than half of the arable land); in 2018, it ranks just behind the United States. Soya occupies two-thirds of the area covered by biotech crops (34.86 million hectares), with the remaining third divided between maize (15.38 million hectares) and cotton (1 million hectares). The proportion of transgenic plants is very high in these three crops: 97% for soya, 89% for maize and 84% for cotton. They are mostly HT, but more gene stacks are being developed (75% of maize and 59% of cotton grown in 2017 are IR-HT). GM sugar cane was introduced in 2018 on 400 hectares. Among the 68 transformation events that have been approved in this country, there are now several stacks that give soybean a double tolerance to two different herbicides (glufosinate and dicamba) or, together with two insect resistance genes, a triple tolerance to three different herbicides (glyphosate, glufosinate and 2-4D), allowing for a variety of herbicide treatments and thus delaying the onset of resistance in weeds10. We should note that 80% of Brazilian soybean exports go to China.
Paraguay and Uruguay, two other Mercosur countries, are small in size but major in terms of biotech agricultural crops. They are both among the top 10 countries growing biotech plants.
In Paraguay, glyphosate-tolerant HT soybean (Roundup Ready®), approved in 2004, is the dominant GM crop and occupies 91% of biotech acreage, ahead of maize (9%) and cotton (marginal). These crops, exploited in 10,000 farms, occupy 62% of the country’s arable land. Paraguayan soybeans are 96% transgenic, with a development in recent years of HT-IR stacking that has increased in popularity from 17% in 2016 to 35% in 2017. As for corn, in 2017 it was 83% IR-HT polyvalent.
In Uruguay, 3,000 farms are growing biotech soybean and corn. Soya accounts for almost all the biotech area under cultivation (1.09 million hectares). The 50,000 hectares of maize cultivated show a 100% adoption rate, which is to say that all Uruguayan maize crops in 2017 were biotech (adoption rate of 85.7% in 2016). In 2017, a decrease in GM crops was observed in Uruguay (-13%), as was the case in other Mercosur countries (-16% in Paraguay, -3% in Argentina). According to the International Service for the Acquisition of Agri-biotech Applications (ISAAA), this reduction is linked to low crop prices and fluctuations in international trade, as well as significant climate events, flooding and especially severe droughts that affected several Latin American countries that year.
In addition to biotech soybeans, corn and cotton, Latin American countries (Bolivia, Mexico, Colombia, Honduras, Chile and Costa Rica) are developing flax, rice, pineapple and blue carnations on small areas for export (Colombia). Between 2016 and 201711, GM crops significantly increased in several countries: in Chile (+23%), Costa Rica (+22%), Mexico (+13%) and Colombia (+7%).
We note that the American continent on the whole is by far the one that has adhered the most to the cultivation of biotech plants.
Vandana Shiva, an important figure in Indian and global environmental and feminist activism, is fighting against new genetic modification technologies.
See Anthony Shelton et al., “Bt Eggplant Project in Banglades: History, Present Status, and Future Direction“, Frontiers in Bioengineering and Biotechnology, vol; 6, article n°106, August 2018.
The sizeable development of GMOs in the Asia-Pacific region
China was the first country to commercialise transgenic plants in the early 1990s with virus-resistant tobacco and tomatoes, followed in 1997 by Bt cotton (IR) to control lepidopteran pests, and GM papaya, resistant to Papaya ringspot virus (PRSV), grown on a small area for human consumption. In 2017, GM papaya culture occupied 7,130 hectares at an 86% adoption rate, and IR cotton crops occupied 2.8 million hectares at a 95% adoption rate, accounting for most of the land farmed for cotton. These two crops alone employ 7 million farmers. China has also approved 64 transgenic events for import or domestic farming of cotton, rapeseed (Argentinean canola), maize, papaya, petunia, poplar, rice, soybean, sugar beet, pepper and tomato. The Chinese authorities’ proactive policy to promote biotechnology has attracted $3 billion in funding for Chinese research institutes and companies to develop biotechnological lines of drought-resistant wheat and maize, disease-resistant rice, soybean with an improved oil profile and increased yields.
India, on the other hand, developed genetically modified cotton to resist insects as early as 2002, but it was only in 2007 that these crops became most prevalent in the field, reaching an adoption rate of 60%, which later rose to 93% in 2017. With an area of 11.6 million hectares in 2018, today India has the largest area of biotech crops across Asia. However, the attitudes towards transgenic plants throughout India are complex. Indeed, this country’s many states have not all adopted the same position towards GMOs and face intense lobbying efforts by powerful anti-GMO movements, of which Vandana Shiva12 is the leading figure. Nevertheless, experimental trials to diversify crops have been carried out with several species: chickpea, mustard, rice, sugarcane and eggplant. Through partnerships with several countries in South and Southeast Asia (Bangladesh, Indonesia, Philippines), several research and field trial programmes are being developed for delayed ripening papaya, insect-resistant cotton and nutritionally improved rice. Neighbouring Pakistan is growing 2.8 million hectares of various types of IR cotton (34 had been approved between 2010 and 2016) and is also developing trials of HT and stacked IR-HT maize.
Six countries in South and Southeast Asia and the Pacific zone are also developing small areas of transgenic crops on less than 1 million hectares: the Philippines cultivates 630,000 hectares of maize; Myanmar, 310,000 hectares of cotton; Vietnam, 49,000 hectares of maize; Australia, 793,000 hectares of cotton and canola; and Bangladesh, 2,975 hectares of eggplant. In 2018, Indonesia began growing drought-tolerant sugarcane on 1,342 hectares.
The trials and tribulations of eggplant cultivation are worthy of our attention. Indeed, the eggplant (also called brinjal) originates from India, which produces more than 13.5 million tons per year, accounting for 25% of world production. Ranked just behind China, India is the world’s second largest eggplant producer. This widespread crop is ravaged by a Lepidoptera that causes crop losses ranging from half to three-quarters of production. To limit this damage, repeated and not-too-distant insecticide treatments (20 to 40 treatments per season in India) must be carried out, but the rudimentary methods employed to apply treatment to the crops are not without consequence for the health of farmers and the environment. This vegetable provides significant supplements (vitamins, minerals, fibre and antioxidants) in the diet of underprivileged populations of the Indian subcontinent and is hugely important for local diets. To find a solution to the insect’s devastation, Mahyco, India’s leading seed producer (at the time 26% owned by Monsanto), in collaboration with the University of Tamil Nadu, developed local varieties of Bt eggplants resistant to the lepidopteran pest. Despite conclusive field trials carried out between 2002 and 2006, the Indian authorities did not grant authorisation to market it under pressure from anti-GMO NGOs. The Indian moratorium of 2010 was followed in 2011 by a halt, for the same reasons, to trials in the Philippines, where eggplant is the most widely grown vegetable. In fact, this promising innovation had been all but buried with one exception. In Bangladesh, one of the poorest countries in the world, where eggplant is the third most important agricultural crop, field trials were carried out for the Bt eggplant varieties developed in India in 2014, with the support of the Ministry of Agriculture: the 20 farmers who grew them on 2 hectares saw their yields increased by 30% and a 30-70% reduction in insecticide treatments. Despite sustained intimidation of producers by anti-biotechnology NGOs, the area dedicated to commercial cultivation of Bt brinjal continued to grow to reach 27,000 farmers exploiting 2,400 hectares of commercial arable land of brinjal, expanded to nearly 3,000 hectares in 2018. A recent study compared 505 farms growing transgenic eggplant with 350 farms producing conventional varieties: it showed a 61% saving in pesticides13 and a six-fold increase in income per hectare for farmers producing Bt brinjal. Bangladesh is currently pursuing a very active policy of adopting this biotech food crop.
See Marcel Kuntz, The Séralini The Dead-End of an Activist Science, Fondation pour l’innovation politique, September 2019 .
Gilles-Eric Séralini, Emilie Clair, Robin Mesnage, Steeve Gress, Nicolas Defarge, Manuela Malatesta, Didier Hennequin and Joël Spiroux de Vendômois, “RETRACTED : Long term toxicity of a Roundup herbicide and a Roundeup-tolerant genetically modified maize“, Food and Chemical Toxicology, 50, n°11, November 2012, p. 4221-4231.
Claudine France, “Biotechnologies végétales et pays en développement”, in Catherine Regnault-Roger, Louis- Marie Houdebine and Agnès Ricroch (eds), Au-demà des Science-Innovation-Société, Presses des Mines, 2018, p.115-134.
The African continent: between doubts and aspirations
While many trials are underway in West Africa – Burkina Faso, Cameroon, Ghana and Nigeria – and East Africa – Kenya, Mozambique, Malawi, Uganda – for, among others, cowpea, maize, sorghum, cotton, sweet potato, rice, banana and soybean crops, only two countries are currently growing biotech crops: Sudan, with 243,000 hectares of IR cotton, and South Africa, with 2.7 million hectares of corn, soybean and cotton (see table page 12). Very recently, a third country on the continent joined their ranks: Eswatini (ex-Swaziland), with 250 hectares of IR cotton planted in 2018.Several trials of transgenic crops have been conducted in some African countries, such as in Burkina Faso (IR cotton) or Kenya (herbicide tolerant cotton project) which were later abandoned:
- in Burkina Faso, some varieties incorporating the transgenic Bt event to control cotton maggot had the defect of producing shorter cotton fibres than conventional varieties. Despite the reduction in pesticide treatments and the increase in yields, the marketing of this cotton crop was less lucrative and Burkinabe farmers abandoned these varieties which did not correspond to their expectations;
- In Kenya, the Séralini case14, in which photographs of rats deformed by tumours were published on the front page of the newspapers made a lasting However, the research article in question15 has since been invalidated by the scientific community and retracted by the journal that published it.
To explain Africa’s ambivalence towards GMOs, Claudine Franche from the Institute for Research for Development (IRD) points to “multiple factors, including the lack of political will to put in place the necessary legislative and legal framework, the risk of seeing the agricultural sector fall under the control of large multinationals, the lack of scientific expertise allowing an in-depth benefit-risk analysis of these crops, and finally the influence of Europe on the continent, where the precautionary principle prevails”16.
One international project, however, is attracting attention: the Water Efficient Maize for Africa (WEMA) project adapting maize cultivation to drought. It is the result of a public-private partnership between the Centro internacional de mejoramiento de maíz y trigo (CIMMYT, “International Maize and Wheat Improvement Center”), which provides high-yielding maize varieties adapted to African conditions, and national agricultural research organisations based in Kenya, Mozambique, Uganda, South Africa and Tanzania, that provide the development infrastructure, as well as the company Monsanto (now Bayer), which is contributing its knowledge of the MON 87460 transgenic event integrated into maize varieties.
First cultivated in the United States in 2013 and in South Africa in 2014, varieties of this transgenic maize have yields that are 7 to 15% higher than traditional crops under water-stressed conditions. Tests to develop gene stacks (IR, better use of nitrogen) are continuing in the African partner countries of this project. Funded by the Bill and Melinda Gates Foundation and the Howard Buffett Foundation, as well as the United States Agency for International Development (USAID), the WEMA project is expected to make royalty-free seed available to African farmers.
See Catherine Regnault-Roger, Des outils de modification…, op cit.
See Bernard Le Buanec, Les Pourquoi la France n’en cultive plus ?, Presse des Mines, 2016.
François Fillon, Faire, Albin Michel, 2015, 137.
See Catherine Regault-Roger, “La réglementation au cœur des débats”, in Catherine Regnault-Roger, Louis- Marie Houdebine and Agnès Ricroch (eds.), cit., p. 135-164.
“Loi n°2014-567 du 2 juin 2014 relative à l’interdiction de la mise en culture des variétés de maïs génétiquement modifié“, Journal officiel de la République française, 3 June 2014, 9208.
See Haut Conseil des biotechnologies (HCB), “Avis en réponse à la saisine du 24 avril 2014 de Messieurs Bernard Accoyer et Jean Bizet suite à la proposition de loi relative à l’interdiction de la mise en culture des variétés de maïs génétiquement modifié sur le territoire français“.
“Directive (EU) 2015/412 of the European parliament and of the Council of 11 March 2015 amending Directive 2001/18/EC as regards the possibility for the Member States to restrict or prohibit the cultivation of genetically modified organisms (GMOs) in their territory Text with EEA relevance”, Official Journal of the European Union, 13 March 2015, 68, L 68/1-68/8.
Catherine Regnault-Roger, “La Réglementation au cœur des débats”, cit., p.156.
The European continent, extraordinary hostility towards biotech
The vast majority of the European continent is very hostile to the cultivation of transgenic plants. Only two European Union countries, Spain and Portugal, are resisting the overwhelmingly discouraging environment. However, although the European Union only grows biotech plants on a very marginal scale, it imports them on a massive scale: it is the world’s second largest importer of soya, after China, 80% of which is transgenic.
In Europe, in 2018, the Iberian Peninsula was home to fields of MON 810 maize, the only transgenic plant cultivated in the European Union, on an area of 120,990 hectares, a significant decrease from 131,535 hectares (124,227 hectares in Spain, 7,308 hectares in Portugal) in 2017. There is very strong psychological pressure in the European Union against GM crops, and imports of maize into Spain from South America and the United States (mostly GM) are very competitive.
MON 810 maize is a Bt (IR) maize that has been grown for more than 15 years in Spain with no incident and to the great benefit of the farmers who plant it. They save on insecticide treatments and the crops contain fewer mycotoxins. Indeed, it is in regions where the European corn borer and Sesamia, two major crop pests, are endemic and exert strong pest pressure, that the MON 810 crops are concentrated (82 % of the total area), namely Aragon, Catalonia and, to a lesser extent, Extremadura. From one year to the next, farmers may choose to grow transgenic maize or return to conventional varieties if pest pressure has temporarily decreased, as was the case in Catalonia during the 2010s. In Portugal, MON 810 is grown in the Alentejo and Lisbon regions, where farms are large enough that they do not interfere with European regulations for the coexistence of agriculture17.
Previously, MON 810 was also grown in the Czech Republic and Slovakia for livestock feed or the biofuel industry, but anti-GM campaigns have hampered its development. There were also transgenic Amflora potato crops, whose starch had interesting properties for the biodegradability of industrial compounds (packaging, adhesives, textiles, etc.) and starch products, produced in Germany, Sweden and the Czech Republic. Authorised in 2010 by the European Commission, these crops were abandoned two years later following the destruction of plots by activists. The BASF Corporation, which marketed this potato, then decided to relocate this activity to the United States in 2012. Since then, several French and foreign companies have followed their lead and moved their biotechnology research and development activities to the American continent (both North and South).
Currently, after a short-lived venture in biotechnologies, most European Union countries reject the cultivation of biotech plants. A reversal of this attitude is due, in part, to various reasons linked to nation-specific contexts, but above all to the relentless action of anti-GMO NGOs such as Greenpeace.
In France, there have been many renunciations of biotechnologies, which Bernard Le Buanec, a member of the French Academy of Agriculture and the Academy of Technologies, has dutifully relayed18. He details a convergence of attitudes that cemented this rejection: that of mass distributors who rely on aggressive marketing strategies centred on GMO-free labelling (Carrefour group); that of public research in the 1980s (INRA and its Executive Director Guy Paillotin) which practised ambiguity; that of the agricultural unions, which have been rather reserved about accepting GMOs, and sometimes explicitly against them (Confédération paysanne); as well as the attitude of certain media that indulge in recurrent misinformation. In addition to this prohibitive environment, the experimental trial crops of cooperatives, official variety evaluation bodies and research institutes are regularly destroyed, and private farmers’ GM crops have often been sacked (when GM maize cultivation was authorised in France, before 2014) by environmental and anti-biotechnology activists, among whom Les faucheurs volontaires, the Confédération paysanne and Greenpeace are particularly prominent. The destruction of such private property is often granted a great deal of judicial leniency, even when property and the rights of the common good are clearly being violated.
From 2008 onwards, a change in attitude of French governments was also starkly apparent as they became hostile to the cultivation of biotech plants on French territory. This reversal is linked to some kind of “deal” made between President Nicolas Sarkozy and the Green Party during the 2007 Grenelle Environment Forum, as recounted, among other accounts, by François Fillon, then Prime Minister:
“He [Nicolas Sarkozy] arbitrated for a total ban on GMOs and open-field experiments […], their abandonment would make it possible to obtain a deal with the ecologists who, if they got this concession from our administration, would not obstruct our plans for nuclear energy”19. The presidential majorities that followed were also attentive to political ecology and based themselves on this “deal” against GMOs, which became a pact that was destined to endure.
The next six years ensued a political and regulatory imbroglio and what I called a “scientific farce” because, until 2015, European regulations did not allow Member State to prohibit the cultivation of transgenic plants authorised by the Community unless a safeguard clause was invoked on grounds that they endangered public health or the environment, a clause that Sarkozy’s government and Dutch presidencies did not hesitate to invoke by producing tendentious and far-fetched scientific arguments, becoming the laughing stock of European expert committees and discrediting the words of scientists and politicians. The High Council for Biotechnology (HCB) was thus carefully kept away from these manoeuvres because its scientific committee was deemed not impressionable enough to participate in such scientific deviations20. The European Food Safety Authority (EFSA), responsible for examining the merits of the French applications, rejected them four times, in 2009, 2011, 2012 and 2014. As a result, the Court of Justice of the European Union (CJEU), following opinions from EFSA, annulled the moratoria imposed by successive French governments, which was subsequently endorsed by the French Council of State.
The climax of this political-scientific farce was the publication, on 2 June 2014, of a law that prohibited the cultivation of genetically-modified maize varieties on French territory21 (based on unfounded scientific arguments, as demonstrated by HCB22), followed by the adoption of a directive amending European regulations and authorising a European Union Member State to ban the cultivation of GMOs for “serious reasons such as those related to environmental policy objectives; land development; land use; socioeconomic impacts; avoidance of GMOs in other products […]; agricultural policy objectives; public order, the latter point to be used in conjunction with another”23 motif. The French governments at the time were thus able to oppose, in complete EU legality, any cultivation of genetically modified plants on national territory. They jumped at the opportunity, putting an end to the cultivation of Bt MON 810 maize, which had been authorised, then later banned again. As I have had the opportunity to point out, the takeaway from this episode is that “the cultivation of genetically modified plants was prohibited in France for reasons of political alliances, and that the European Commission has accepted that regulation should no longer be based solely on scientific criteria but on the political and ideological positions of the rulers and governments of each Member State, thus leaving the field open to subjectivity and arbitrariness”24.
Developing countries lead in biotech crops
In 2018, 28 countries were cultivating GMPs. While 42 other countries, such as France and most of the European Union Member States refused to cultivate them, they were nevertheless heavily importing biotech products. A total of 70 countries accept transgenic products and commercialise them.
This observation leads us to conclude that over the last thirty years, this technology has been one of the most rapidly adopted in the world. This is all the more remarkable as it is not only being utilised by developed countries, but also and mostly by developing countries that quickly surpassed them. It was in 2010 that their growth reached the same level. Eight years later, developing countries were cultivating more than 100 million hectares of biotech crops, or 54% of the land area (46% for industrialised countries). Every year, the amount of biotech land under cultivation in developing countries increases significantly.
Contrary to what is often suggested, large farms in North America or haciendas and fazendas in Latin America are not the only ones growing transgenic plants. They are frequently cultivated by millions of small farmers, particularly in China and India: the headcount has increased from 12 to 17 million between 2007 and 2018, 90% of whom have very low incomes. Among the arguments in favour of GMPs put forward by these small farmers, the most common ones are: less arduous work, better protection of their health by reducing painful and frequent applications of phytosanitary products on crops and better income linked to increased yields.
A race for innovation in a heterogeneous regulatory world
Genome editing organisms (GEOs): the race for innovation and patenting
See Dominik Modrzejewski et , “What is the available evidence for the range of applications of genome editing as a new tool for plant trait modification and the potential occurrence of associated off target effects: a systematicmap”, Environmental Evidence, vol. 8, article n°27, July 2019.
See Catherine Regnault-Roger, Des outils de modification…, op. cit.
See Jacqueline Martin-Laffon, Marcel Kuntz and Agn-s Ricroch, “Worldwide CRISPR patent landscape shows strong geographical biases”, Nature Biotechnology, vol. 37, N°6, June 2019, pp. 613-621.
It is in this context of a world divided on the acceptance of biotechnologies, that a new race to innovate and register patents for products resulting from new genome-editing techniques, the EMBs, is taking place today.
The numbers already speak for themselves. Thanks to two recent publications, it is possible to compare the dynamism of research publications on NPBTs by country25 and look at the patents on Clustered Regulatory Interspaced Short Palindromic Repeats (CRISPR) technology26 that have been filed over the last six years27.
Geographical distribution of NPBT research publications and CRISPR patents between 1996 and 2018
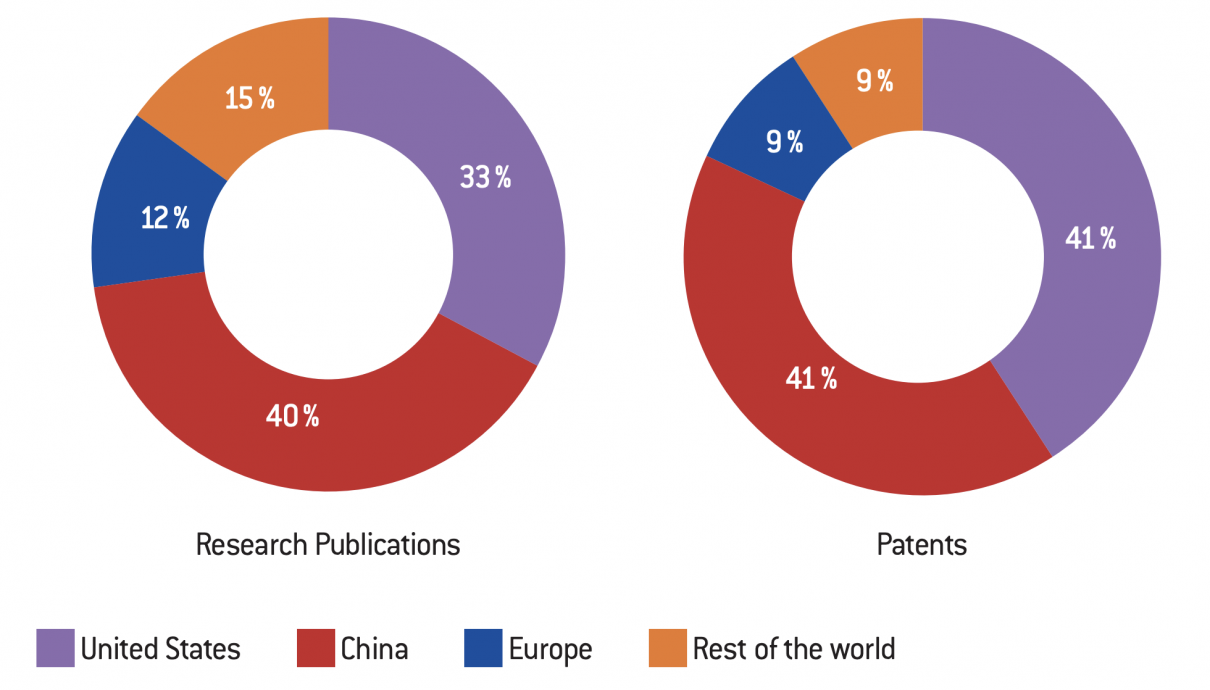
The research work accounted for between 1996 and May 2018 demonstrates the dynamism of Chinese and American laboratories in this new sector and a survey of patent applications for the period 2012-2018 further supports this conclusion. The two most prolific countries are undoubtedly China and the United States, both in terms of improved techniques and applications. However, while U.S. laboratories remain leaders in technical improvements and medical applications, China has filed the most patents in the industrial and agricultural (plant and animal) sectors. Applications in the plant sector account for 13% of the total patents listed.
Geographical distribution and sectors of applications of CRISPR patents filed between 2012 and 2018
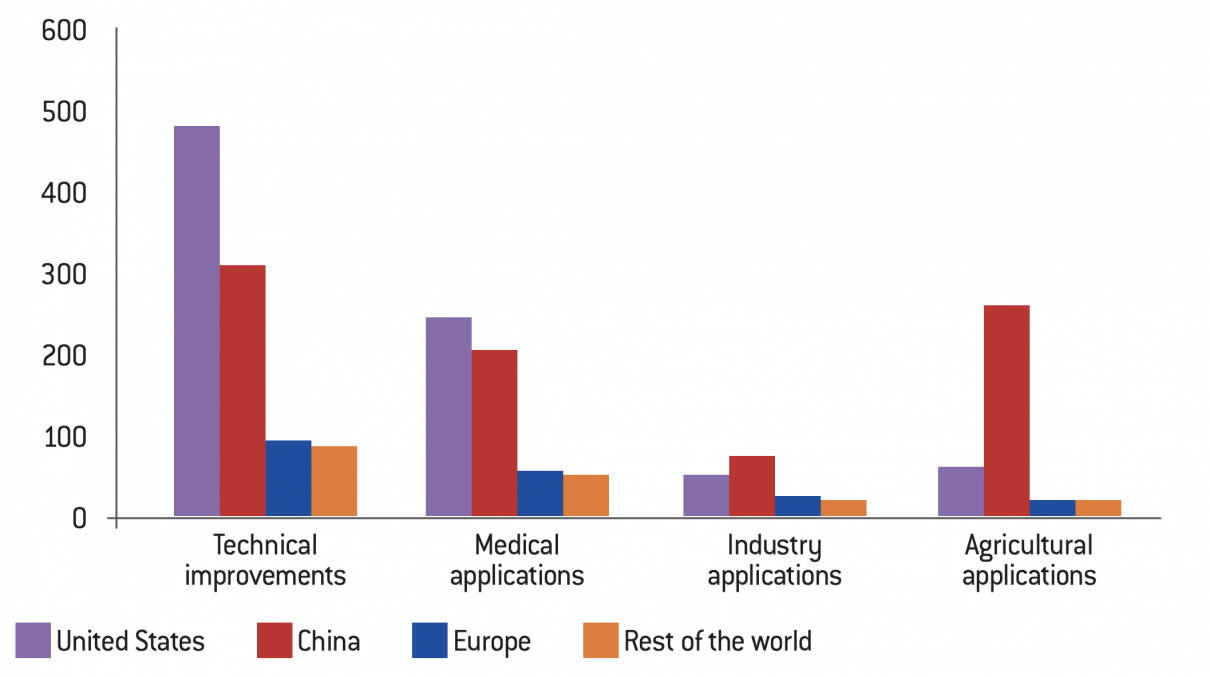
Source :
Based on Jacqueline Martin-Laffon, Marcel Kuntz and Agnès E. Ricroch, “Worldwide CRISPR patent landscape shows strong geographical biases”, Nature Biotechnology, vol. 37, No. 6, June 2019, pp. 613-621.
See Arnd Hoeveler and Etienne Magnien, “Quand les instances européennes mènent le bal”, Biofutur, n°172, November 2017, 12-15.
See Jean-Claude Pernollet, “Plantes génétiquement modifiées”, in Catherine Regnault-Roger (ed.), Idées reçues et agriculture. Parole à la science, Presse des Mines, 2018, p.188-204.
We can see that Europe is lagging behind. With 9% of patents filed and 13% of research publications, it is on par with all other countries in the world combined (Australia, Japan and Latin American countries, with Africa being very marginal), far behind the two leaders. This technological delay will undoubtedly have economic and geopolitical implications for the future.
Do you remember that between 1989 and 1994, the European continent dominated biotechnology innovation? During this time, European companies had filed more patents on biotechnology than American companies (203 versus 178), as two senior European Commission officials pointed out at the time28.
This European backwardness in scientific production, along with a dearth of intellectual property, is likely to aggravate in coming years if the regulatory attitudes of nations do not evolve, with some in favour of the development of biotechnology while others remain staunchly against it, and have devastated consequences on the state of their agriculture. Jean-Claude Pernollet, a member of the French Academy of Agriculture, thus emphasises that “the perennial deadlock in the debate on GMPs is particularly harmful to the agricultural future of countries that refuse to cultivate them29“. The regulations, not only for GMOs but also those that will be applied to GEOs, are at the heart of a serious geopolitical issue.
European regulations steeped in the principle of precaution
See Claudine franche, “Biotechnologies végétales et pays en développement”, op. cit.
Directive 89/219/EEC, 7 March 1989.
Directive 2001/18/EC, 12 March 2001.
Directive 2015/412, 11 March 2015.
Directive 2018/350, 8 March 2018.
Regulation 2015/2283, 25 November 2015.
Elements of the Regulatory Environment
Across the globe, regulations on transgenic plants are not homogeneous. Two logics face one another: On the one hand, Bangladesh, Colombia, Costa Rica, Honduras, India, Mexico, the Philippines, Sudan and Uruguay base their regulations, like Canada and the United States, on the characteristics of the products obtained; on the other hand, Australia, Bolivia, Burkina Faso, China, India, Pakistan and South Africa, like the European Union and New Zealand, base their regulations on the techniques used to produce biotech plants30. This difference in regulatory currents has implications for risk assessment of GMOs and for the management of marketing authorisations granted by governments.
Traditionally, regulations in the field of public and environmental health are intended to protect people or their environment from harmful effects on their health. It plans and acts based on tangible and reliable assessments to manage risks, i.e. to limit exposure to the hazard if it exists, which requires defining what the hazard under consideration would be and its occurrence (directly related to the exposure of the persons to be protected) in order to fully assess the actual risk.
At the end of the 20th century, transgenesis was then the newest technique for modifying the genome, and opened the exploration of new fields of knowledge and immense possibilities for its applications.
To face these innovations, although the outlook was still poorly defined, the scientific community took to assessing the risks that could be associated with this new technology. Organised by Paul Berg, a biochemist at Stanford University and future Nobel Prize winner in chemistry in 1980, a scientific debate was held in 1975 at a conference with 150 specialists in Asilomar, California, which led to the establishment of regulations on GMOs in various countries. In the United States, the Coordinated Framework for Regulation of Biotechnology was published in 1986. In France, that same year, the Commission de Génie Biomoléculaire (CGB) was created and charged with analysing potential health and environmental risks related to GMO experimentation in an open environment. This was followed by the introduction of European regulations on GMOs in several stages: firstly, a set of two directives articulated together and published in 1989 and 1990, relating to the use of GMOs in contained or open environments31, then, ten years later, in 2001, a directive “on the deliberate release of GMOs into the environment”32, still in effect but amended in 201533, and lastly, in 2018 a directive updating the regulatory framework of provisions for monitoring environmental risk assessment34. In 2015, a new Novel Food Regulation was also published to accompany these guidelines35.
Based on this principle of precaution, the legal corpus of the European Union is increasingly demanding and restrictive for the use of GMOs (cultivation and imports) on European territory and is somewhat related to the current European situation where most Member States distrust this technology. Denials of authorisation are now mostly based on “negative public opinion”.

A deoxyribonucleic acid molecule created in the laboratory by genetic engineering.
Christine Noiville and Estelle Brosset, “Les nouvelles techniques d’édition du génome donnent-elles naissance à des OGM couverts par la directive 2001/18 : la Cour de justice de l’Union dit deux fois oui !”, Cahiers Droit, Sciences & Technologies, n°8, 2018, 17.
Ibid. §19. This is a question put forward by a national court when the resolution of a dispute submitted to it is conditioned by the interpretation of a rule of European Union law.
As quoted in Jean-Baptiste Jacquin, “Tensions entre le Conseil d’Etat et la Cour de justice de Luxembourg”, Le Monde, 17 May 2019.
In addition to this general overview, there is one final legal twist: the European Court of Justice (ECJ) ruling from 25 July 2018, which ruled that products obtained by mutagenesis techniques subsequent to Directive 2001/18/EC (targeted mutagenesis) must be subject to GMO regulations, whereas those obtained by “traditional” mutagenesis techniques (i.e. used before 2001) may continue to be exempt from these regulations, although Member States are free to subject them to these regulations if they so choose. This court decision does not follow the conclusions of Advocate General Bobek, published on 18 January of the same year, who had favoured the use of recombinant DNA36 in his analysis. On the other hand, it broadens the scope of Directive 2001/18/ EC to include products obtained by genome editing (GEO) as they are the result of techniques applied after 2001 and implemented by man, i.e. in an “unnatural” way. This means that GEO varieties should be subject to the European assessment and labelling procedures for GMOs.
In making this ruling, the CJEU has made “a radical choice, very legally convincing, in line with the precautionary imperative imposed on the Union”, according to legal experts Christine Noiville and Estelle Brosset37.
They nevertheless specify that “in the context of a preliminary ruling, it is not for [the CJEU] to judge the facts, but rather to interpret Union law”38 and that it is up to the political authorities to decide what the conditions are for “proven safety” linked to the use of GMOs to be met …, which inevitably refers to the interpretation of the principle of precaution and the lock that it constitutes.
In France, the Conseil d’Etat (Council of State) has not always acted upon this judgment, but its margin of manoeuvre is narrow because, as Louis Boré, President of the Bar Association at the Council of State, states: “The relationship between the Council of State and the Court in Luxembourg is that of a dialogue between a teacher and his pupil, but, in the event of a power struggle, it is always Luxembourg or Strasbourg that will prevail”39.
Directive 2001/18/EC, op. cit.
Formore informationonthispseudo-debatebetweentheartificial andthenatural, see Catherine Regnault-Roger, Des outils de modification…, op.cit.
For more information on transgenesis, see ibid.
See EFS, “Guidance on the Post-Market Environmental Monitoring (PMEM) of genetically modified plants”, EFS Journal, vol. 9, n°8, August 2011, p.2316-2247 (https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j. 2011.2316).
See Catherine Regnault-Roger, Des plantes biotech…, cit.
French Variety and Seed Study and Control Group (GEVES), “The official catalogue of Species and Varieties of Cultivated Crops in France“.
See European Commission, “Plant variety catalogues, databases & information systems”.
See Jeremy Rifkin, Le siècle biotech, French edition, La Découverte, 1998.
Cumbersome and costly European regulation on GMOs
Directive 2001/18/EC defines in its Annex 1 what a GMO is and details the techniques that will be subject to regulation, i.e. those that will be considered as producing regulated GMOs and those that will be excluded from the scope although they cause genetic modification (mutagenesis, cell fusion of plant cells “which may exchange genetic material by traditional breeding methods”40). The determining criteria to be within the scope of the regulation are the use of techniques that result in the unnatural incorporation of genetic material produced or prepared outside the organism whose genome is to be altered, or unnatural methods that arrange cell fusion with new combinations of genetic material41. Transgenesis42 techniques are thus subject to Directive 2001/18/EC.
In order to be granted GMO commercialisation authorisation, many applications must be filled out. Each section must be completed in great detail and require a significant investment in terms of laboratory or field experiments in order to provide the answers requested. Moreover, when an application for a permit for Europe (import or cultivation) is submitted, it is not always easy for the petitioner to have relevant data adapted to the European flora and fauna because of the frequent destruction of field experiments by anti-GMO activists. The flora and fauna data collected in countries where transgenic plants are more reliably cultivated (on the American continent) are often inapplicable to European countries.
The technical file must include a full description of the plant and its transformation including a complete toxicological report (food safety, allergenicity, etc.). It is must be accompanied by an environmental risk assessment plan specifying the exposure and the potential existence of gene flows, persistence and invasion phenomena, possible immediate, delayed or cumulative long-term effects on target and non-target organisms (fauna, flora of the environment, operators), on biogeochemical cycles and what measures are taken to manage these risks.
Finally, it must conclude with a post-commercialisation surveillance plan, which has two components:
- General monitoring, intended to reveal unpredictable changes and unknown unintended effects on non-target populations: this is known as non-hypothesis- driven This approach is a perfect illustration of the pusillanimous application of the principle of precaution;
- Specific monitoring, designed to highlight the occurrence of expected changes and to test possible hypotheses for adverse effects of the transgenic plant related to its use in the environment.
Post-market monitoring of a transgenic plant is conducted over the ten-year duration of the authorisation and gives rise to annual reports sent to the European Commission via EFSA, which has them assessed by the Member States before issuing conclusions. Since 2011, EFSA has been requesting that the post-marketing monitoring plan include studies on virtual critical situations and risks evaluation in iterative scenarios43. In the case of imports, general surveillance is also carried out to detect anomalies between the places of transhipment and delivery of cargoes.
This considerable effort is very expensive (more than 100 million euros per file according to companies filing applications) only to detect an uncertain and unknown abnormality that could potentially be related to the cultivation of a transgenic plant. Only the large international conglomerates in the sector which have been formed through mergers and acquisitions over the last 20 years (Corteva AgriScience, ChemChina and its subsidiary Syngenta, Bayer, which absorbed Monsanto44) have the financial strength to meet such specific regulatory requirements.
In addition to this specific dossier for GMOs, the normal procedure to petition marketing authorisation (MA) for a new plant variety still applies. Indeed, when a new variety is obtained, for example selected for a better resistance to a harmful agent (insect pests or pathogens) whether by classical crossing, mutagenesis or transgenesis, it must be registered in the “Official Catalogue of Plant Species and Varieties Cultivated in France”45 to be marketed. These catalogues have been drawn up to protect the buyer (farmer, market gardener, horticulturist, gardener, etc.) by guaranteeing the varietal conformity and quality of the seeds that are sold.
In order to be marketed, a new species or variety of plant must pass the assessment tests carried out by the Variety and Seed Study Group (GEVES), which consist of verifying not only the distinctness, uniformity and stability (DHS tests) of the new variety, but also its agronomic, technological and environmental value (VATE tests). It is a demanding procedure: out of about 1,000 candidate varieties tested each year by the Geves, only 30 to 40% pass the DHS-VATE tests. It is carried out under the authority of the Standing Technical Committee on Plant Breeding (CTPS), which in turn submits new varieties to the Ministry of Agriculture for inclusion in the catalogue. It is the Ministry that ultimately grants commercialisation authorisations.
In the summer of 2019, there were more than 9,000 varieties for 190 species listed in the French catalogue, and more than 18,200 varieties of agricultural species and 16,200 varieties of vegetable species listed in the European catalogue, which includes the catalogues of every Member State of the European Union and certain countries of the European Free Trade Association (EFTA). As such, as of 24 June 2019, there are 5,479 varieties of maize alone registered in the Official European Catalogue of Plant Varieties, 150 of which carry the transformation event MON 81046, currently the only transgenic maize grown in the European Union.
Under these circumstances, it is hardly surprising that so few companies are able to bear the costs of marketing GMOs. Was it legitimate to impose this restrictive and costly regulation with so little perspective on this technology? The American essayist Jeremy Rifkin provides the cynical response that the GMO regulations were first established as a safeguard against damages lawsuits in the United States47. Regardless of their purpose, nowadays, many are calling to ease up on these regulations as no major adverse effects have been observed in any of the countries growing biotech plants over the last twenty years.
These calls for change are all the more relevant now that, on 16 May 2016, the National Academies of Sciences-Engineering-Medicine, which brings together national academies of science, engineering and medicine, published a 600- page report entitled Genetically Engineered Crops. Experiences and Prospects. Under the direction of Professor Fred Gould, twenty authors from the academic world contributed a considerable amount of work, analysing more than 1,000 scientific publications on crops produced by genetic engineering over the last twenty years, interviewing 80 actors and collecting 700 comments. The committee examined at length the agronomic and environmental effects of the cultivation of genetically modified crops, and subsequently looked at their effects on public health and examined the social and economic consequences of these crops. This work shows that biotech plants cultivated in accordance with good agricultural practices do not present any more toxicity or ecotoxicity, i.e. health and environmental risks, than conventional plants. On the contrary, they can improve health and environmental safety as well as yields. The report concludes by stating that, in view of the societal debate, the issue of GM plants cannot be examined solely through the prism of science but must take citizens’ expectations into account.
See European Commission, “Commission launches new mechanism to strengthen scientific advice for policy making”.
“A scientific perspective on the regulatory status of products derived from gene editing and the implications for the GMO Directive”, Statement by the Group of Chief Scientific Advisors, 13 November 2018.
United States Department of Agriculture (USDA), “USDA Proposes New SECURE Biotechnology Regulations to Protect Plant Health and Promote Agricultural Innovation“, gov, 6 June 2019.
See “L’Australie et le Japon définissent les règles pour les nouvelles techniques génétiques (NBT)“, seppi. over-blog.com, 19 May 2019.
See Claudine Franche, “Biotechnologies végétales et pays en développement”, art. cit.
What regulations for GEOs?
The issue of a revision of European GMO regulations is all the more relevant today now that, according to the CJEU ruling of 25 July 2018, it should apply to new genome editing techniques. Consequently, this judgment was commented on by the group of senior scientific advisers that make up the Scientific Advice Mechanism (SAM).
On 9 June 2015, the SAM was tasked by the European Commission with providing independent and transparent information on scientific subjects in order to develop EU policies in accordance to scientific fact. The SAM was asked to “bring together evidence and insight from different disciplines and approaches, take into consideration the specificities of EU policy making, and ensure transparency”48.
In a statement49, the SAM stresses that “the GMO Directive [2001/18/EC] states that ‘the regulatory framework for biotechnology should be reviewed so as to identify the feasibility of improving the consistency and efficiency of that framework’”. It also considers that the content of the Court’s judgment clearly shows that, because of “new scientific knowledge and recent technical developments have made the GMO Directive no longer fit for purpose”. The SAM notes that “the impossibility of distinguishing between spontaneously occurring mutations and different types of human interventions is a major issue from a regulatory point of view” and emphasises that “the features of the final product itself must be examined regardless of the underlying technique used to generate that product”. This high-level scientific council is strongly recommending a review of European regulations applied to GMOs, considering them to be unsuitable for NTBs, particularly with concerns of control and traceability. It also stresses the need to assess the characteristics of the final product and not to legislate on the basis of the method of production. It clearly states that the modification of existing European regulations must take into account “current knowledge and scientific evidence, in particular on gene editing and established techniques of genetic modification” and that “this should be done with reference to other legislation relevant to food safety and environmental protection”. In order to create a regulatory environment conducive to innovation and to ensure that “society can benefit from new science and technology”, it calls for societal dialogue between all stakeholders and the general public.
Currently, several countries are in the process of revising their regulation of biotech plants:
- In the United States, the USDA has held a public consultation (currently underway) indicating that its proposal for “sustainable, ecological, consistent, uniform, responsible and efficient” (SECURE) must modernise biotechnology regulation “with a balanced approach that continues to protect plant health while allowing agricultural innovation to thrive”50;
- In Japan, two cases emerge: either the final product contains recombinant DNA and will be subject to GMO regulations, or it does not contain recombinant DNA and will therefore not be considered a GMO;
- Australia will not regulate the products of genome modification resulting from certain techniques where the final product is characterised by the absence of an exogenous DNA sequence51;
In developing countries, Argentina and Brazil are the most advanced in this regard:
- Argentina has opted for a case-by-case analysis; a final product without transgenes should not be subject to the regulation of GMPs, but specific monitoring could be carried out if plant characteristics could give rise to health or environmental risks;
- Brazil will carry out case-by-case assessments that will examine the characteristics and safety of the final product: in this framework, the Comissão Técnica Nacional de Biossegurança (CTNBio) will be able to exempt new Genome edited products from regulatory assessment of GMOs52.
High stakes: power and progress in the battle for social acceptability
On this subject, see Eddy Fougier, Contester les technosciences: leurs raisons, Fondation pour l’innovation politique, July 2011 and Sylvain Boulouque, Contester les technosciences ; leurs réseaux, Fondation pour l’innovation politique, July 2011.
See Hervé Kempf, La guerre secrète des OGM, Seuil, 2003.
See Bernard Le Buanec, op. cit.
Marcel Kuntz, GMOs, the political question, PUG, 2014, p. 17.
As quoted in Bernard Le Buanec, op. cit.
Regnault-Roger, Nouvelles biotechnologies agricoles : l’expertise scientifique au cœur du débat sociétal, Revue de l’Académie d’agriculture de France n°10, pages 38-43 (2016).
GMOs and GEOs are proving to be important tools for preserving the health of humans, animals, plants and the environment, all of which are part of the One Health concept. As with any new product, GEOs will go through phases of technological development that inevitably accompany the beginnings of innovations. Nevertheless, they are one of the keys, though not the only one, to improving health initiatives and adapting, in terms of agriculture, to climate change.
The social acceptability of biotechnologies in the field of public health does not give rise to the polemics that rage in its agricultural applications. The evolution of these biotech crops in the different countries of the world and the regulations applied to them are at the heart of important geopolitical development issues.
The rejection of biotech crops on national territories in France and many European Union countries is already affecting the competitiveness of European agriculture. This policy should not be extended to new biotechnologies, otherwise Europe, which has already fallen far behind the United States and China, will be out of the race for good, suffering with it all the socioeconomic consequences and other dependencies, including in the scientific and political realms.
The rejection of GMOs and genome-editing techniques professed by some NGOs and other environmental lobbyists is not based on scientific arguments. Asking them to look back over the past twenty years and convince them that the benefits outweigh the risks is, however, unrealistic. The NGOs that are fierce opponents of the development of biotechnologies clearly display an ideological and political opposition to contemporary society, and their ideological choice was made very early on and from the beginning53.
As early as 1986, after the financial health of Greenpeace was threatened by the cessation of French nuclear tests at Mururoa and by its positions on the Gulf War54 (more than 1 million donors cut off their contributions to the movement), an informal group gathered in Europe around Isabelle Meister and Arnaud Apotecker decided “that the fight against GMOs would be their new key theme”55. The group decided to launch a campaign against the import of American transgenic soybeans into Europe. The daily newspaper Libération on 1 November 1996 ran the headline “Alerte au soja fou” (“Mad Soy Alert”). This title was a reference to the mad cow crisis that Europe was experiencing at that time and which was, according to Marcel Kuntz, “the determining crisis on the fate of GMPs in Europe”56. While the series of scandals in the 1980s and 1990s (the contaminated blood affair and mad cow disease, among others) undermined public confidence in the public authorities responsible for ensuring health safety, the French management of Greenpeace was not convinced that GMOs were a health hazard. Their mistrust lay elsewhere. Bernard Le Buanec reported the words of Bruno Rebelle, director of Greenpeace France, in 2002: “We are not afraid of GMOs. We are simply convinced that they are a bad solution. GMOs may be a wonderful solution for a certain kind of society. But that is a kind of social project that we do not want”57.
Fifteen years later, anti-biotechnology NGOs are still defending this point of view. In 2016, the Les Amis de la Terre association declared in 2016, on the topic of NBTs58 that: “The choice that will be made about new techniques will decide for or against a societal model that, in a technological rush, seeks to cure the ills of the unbridled consumer society and climate change”. She adds that does indeed reject innovation and invokes the principle of precaution: “The decision that must be made about new techniques is a political choice, not a technical one. Are we maintaining the precautionary principle or are we embarking on a blind and unbridled race that seeks salvation in novelty and a blind faith in progress?”. What is at issue here is not technology, but the society that develops it.
The debate is now clear. GMOs and GEOs, and more generally biotechnological advances and the progress they foster to feed a steadily growing human population (2 billion more human beings between now and 2050, i.e. an estimated world population of 9.7 billion people), to improve the living standards and conditions of developing countries and their low-income populations that remain majorly agricultural, but also to adapt to climate change by promoting innovation, are not in question. Anti-biotechnology pressure groups do not act on scientific and technical criteria but reject biotechnology out of political ideology.
Sylvie Brunel, Toutes ces idées qui nous gâchent la Alimentation, climat, santé, progrès, écologie…, JC Lattès, 2019.
What future do we want for humanity? Should we all subscribe to the prevailing “collapsologie” (an idea studying the risks of a collapse of industrial civilisation and what might succeed today’s society), “this science of disaster, a recipe for disaster that demobilises rather than galvanises us to seek lasting solutions” as Sylvie Brunel describes it59.
Echoing the Rabelais quote at the beginning of this study, we must, more than ever, ask ourselves whether it is possible to believe, in all conscience, in a future without science.














No comments.